Discover our Products in
U.S. Clinical Trial
Clinical trials are research studies that are designed to determine if a medicine is safe and effective for patients.
Phase 2 Study to Evaluate the Efficacy and Safety of CBT-001 in Patients with Pterygium
Condition: Hyperemia Reduction and Pterygium Regression
Phase: Phase II
The objective of the study was to evaluate the safety, efficacy, and pharmacokinetics of CBT-001 ophthalmic solutions (0.02%, 0.05% and 0.2%) in reducing pterygium vascularity and inhibiting legion growth. CBT-001 administered three time daily (TID) for 4 weeks had an excellent safety profile when used in pterygium patients. CBT-001 administered TID for 4 weeks was more effective than the vehicle in reducing pterygium vascularity and inhibiting legion growth.
We had a successful EOP2 Meeting with FDA. FDA agreed with proceeding to Phase 3 trials, agreed on Phase 3 study design and efficacy endpoints, and agreed on CMC, non-clinical and clinical plan. Please check ClinicalTrials.gov to learn more about CBT-001 trial.
Phase 2 Study to Evaluate the Efficacy and Safety of CBT-006 in Patients with MGD
Condition: Meibomian Gland Dysfunction Associated Dry Eye Disease
Phase: Phase II
The objective of the study was to evaluate the safety, tolerability and efficacy of CBT-006 in comparison of vehicle when instilled three times a day (TID) dosing for 3 months in patients with MGD-DED. Please check ClinicalTrials.gov to learn more about CBT-006 trial.
Phase 2 Study to Evaluate the Efficacy and Safety of CBT-004 in Patients with Pinguecula
Condition: Vascularized Pinguecula
Phase: Phase II
The objective of the study was to evaluate the safety, tolerability and efficacy of CBT-004 ophthalmic emulsion when instilled twice daily (BID) for 28 days in patients with vascularized pinguecula. Please check ClinicalTrials.gov to learn more about CBT-004 trial.
Study Design
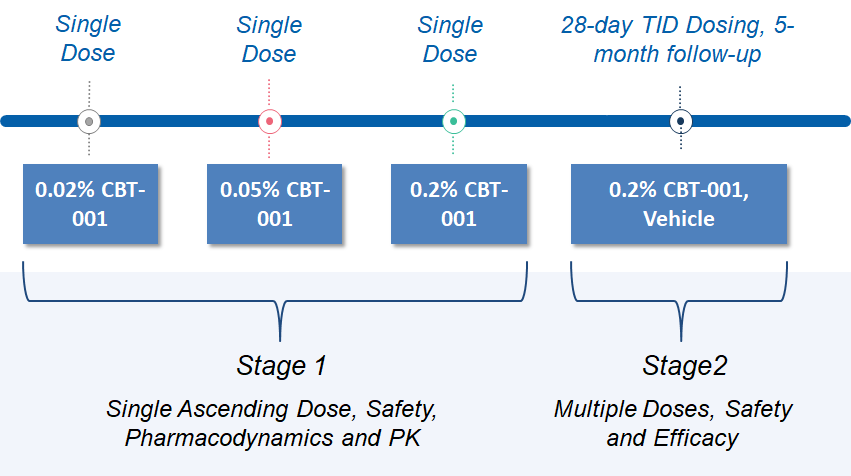
Please visit clinical trials.gov website to learn more about CBT-001 trial, and our Patient Information page to learn more about pterygium.
Primary Endpoint was Met with Highly Statistical Significance
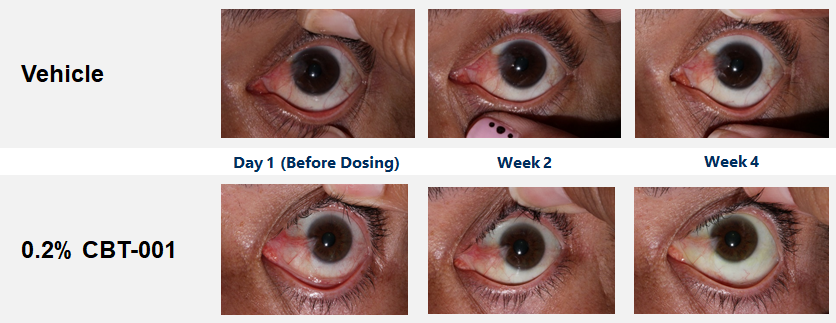
CBT-001 demonstrates good ocular and systemic safety, quick onset, and highly effective during and post-dosing.
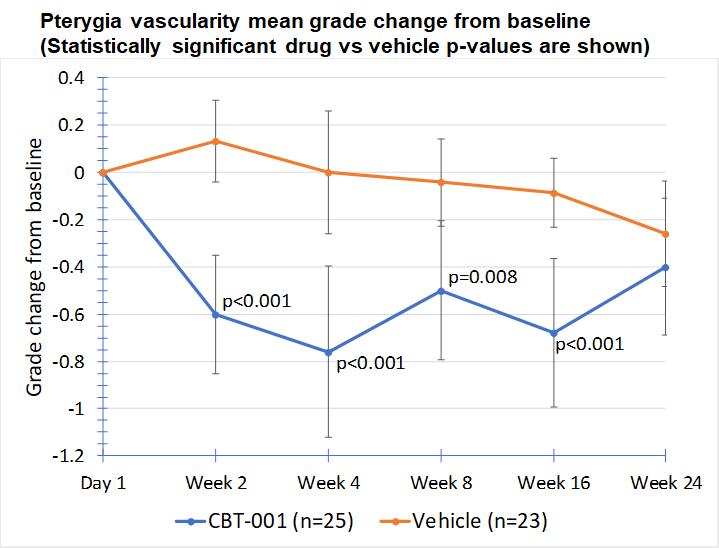
Baseline demographic characteristics were similar between patients receiving CBT-001 (n=25) and vehicle (n=23). After four (4) weeks of dosing, mean vascularity scores were significantly decreased in patients receiving CBT-001 (-0.8) compared to vehicle (0.0) (p<0.001). Vascularity remained significantly decreased at weeks 8 (p=0.008) and 16 (p<0.001), but not at week 24. The CBT-001 group showed significantly greater mean reductions in lesion length at week 2 (p=0.005), week 4 (p=0.007) and week 8 (p=0.0145).
EOP2 Meeting with FDA
Proceed with Phase III Trials after a Successful EOP2 Meeting with FDA Regarding CBT-001 as a Treatment for Pterygium
We had a successful EOP2 meeting with the FDA. The FDA agreed with proceeding to Phase III trials, agreed on Phase III study design and efficacy endpoints, and agreed on CMC, non-clinical, and clinical plan.
