Our Phase III Ready Drug Candidate, CBT-001, Would be the Disease-Modifying, First-in-Class, and First Drug Therapy to Treat Pterygium
Breakthrough Innovation
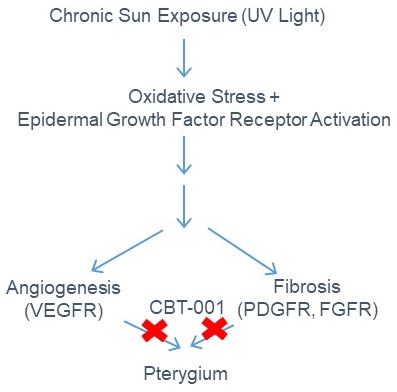
CBT-001 is a potent multi-kinase inhibitor of VEGFRs, PDGFRs, FGFRs, among other targets and can inhibit angiogenesis & fibrosis. CBT-001 is a unique topical formulation following 505b(2) regulatory pathway to treat pterygium that currently has no approved drug treatment. Surgical excision is the only option and the standard of care for pterygium patients. The angiogenic and fibrotic pathogenesis of pterygium is well established. By targeting these pathways pharmacologically, we will stop pterygium progression and eliminate the need for excision surgery.
Efficacy and Safety
In the Phase II clinical trial, CBT-001 has demonstrated highly significant in reducing pterygium vascularity, cornea lesion length, and conjunctival hyperemia. In addition, CBT-001 is well tolerated in both the treated eye and systemically. No patient withdrew due to adverse effects. There are no serious drug reactions or adverse effects reported. We have a successful EOP2 Meeting with FDA. FDA agreed with proceeding to Phase III trials, agreed on Phase III study design and efficacy endpoints, and agreed on CMC, non-clinical and clinical plan.
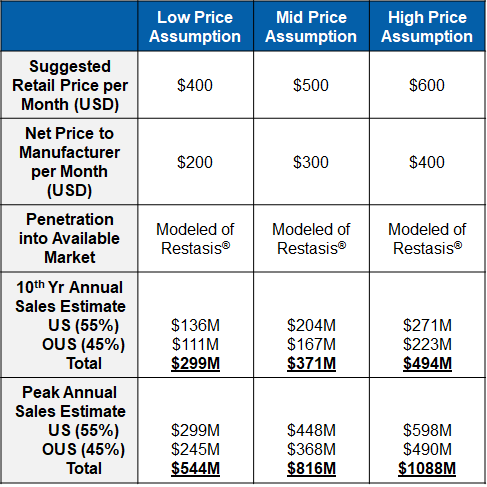
Market research supports a robust sales forecast for CBT-001 for the following reasons:
- CBT-001 addresses a largely unmet medical need
- It is a first-in-class and the first drug therapy to treat pterygium
- CBT-001 is a disease modifying therapy to regress pterygium size and reduce hyperemia
- It has quick onset for the treatment
- Its effect sustains for a long period
- It is a convenient self-dosing eye drop
- CBT-001 is an early treatment to avoid surgery or reduce recurrence rate post-surgery.